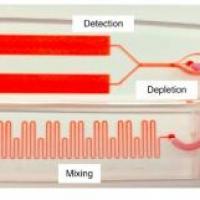
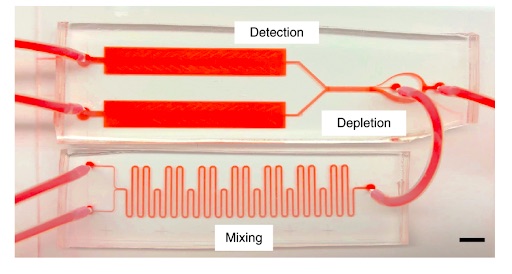
Photo: Mahla Poudineh
Stanford researchers have developed a new biosensor with the ability to continuously detect and monitor the blood concentration of insulin and glucose in real time. The system, dubbed the Real-time ELISA or RT-ELISA, is a breakthrough in continuous patient monitoring technology and could revolutionize the way doctors treat disease.
Researchers in Tom Soh’s bioengineering lab have expanded on the existing technology of an ELISA, a test often used to detect the presence and concentration of a particular antibody, hormone or other compound in a blood sample. Current ELISA technology is not able to run continuously; it can only represent a “snapshot” of what was happening in the body at the instant that the blood sample was drawn. The results are also not instantaneous, and take between one to four hours (or sometimes overnight) to process, according to Caitlin Maikawa, Ph.D. ’21 in bioengineering and co-lead author on a recent study involving the RT-ELISA published in Nature Biomedical Engineering.
By contrast, the RT-ELISA can deliver results instantly and reports changes in blood chemistry over time, providing “movies” in lieu of “snapshots.” According to the study, the device works continuously to draw a tiny amount of blood from a patient and pass it through a microchip. Inside this microchip, the blood is mixed with tiny beads which are studded with antibody or aptamer proteins that latch onto the compound of interest. Once this target compound has been caught, the bead begins to glow due to fluorescent tags which are excited by a laser. When more of the target compound is present in the blood, more beads glow. A high-speed camera instantaneously reports the concentration of the target substance by measuring the amount of light emitted from the sample.
Another important feature of the RT-ELISA is its adaptability. In the past, the few continuous monitoring systems available were built to detect only a specific target molecule like glucose or lactate. In contrast, the microbeads in the RT-ELISA, which are currently used to snare glucose and insulin, could be altered to capture different targets. According to Maikawa, it’s likely that almost any substance for which researchers currently have an antibody or aptamer “could be easily subbed into this platform and measured.”
Forging direct insulin monitoring capabilities
The RT-ELISA could also allow diabetic patients to “watch” their body process insulin and tailor their dosing regime to suit their body’s individual needs.
According to the study, the RT-ELISA was recently used to continuously detect the presence of glucose and insulin in rats.
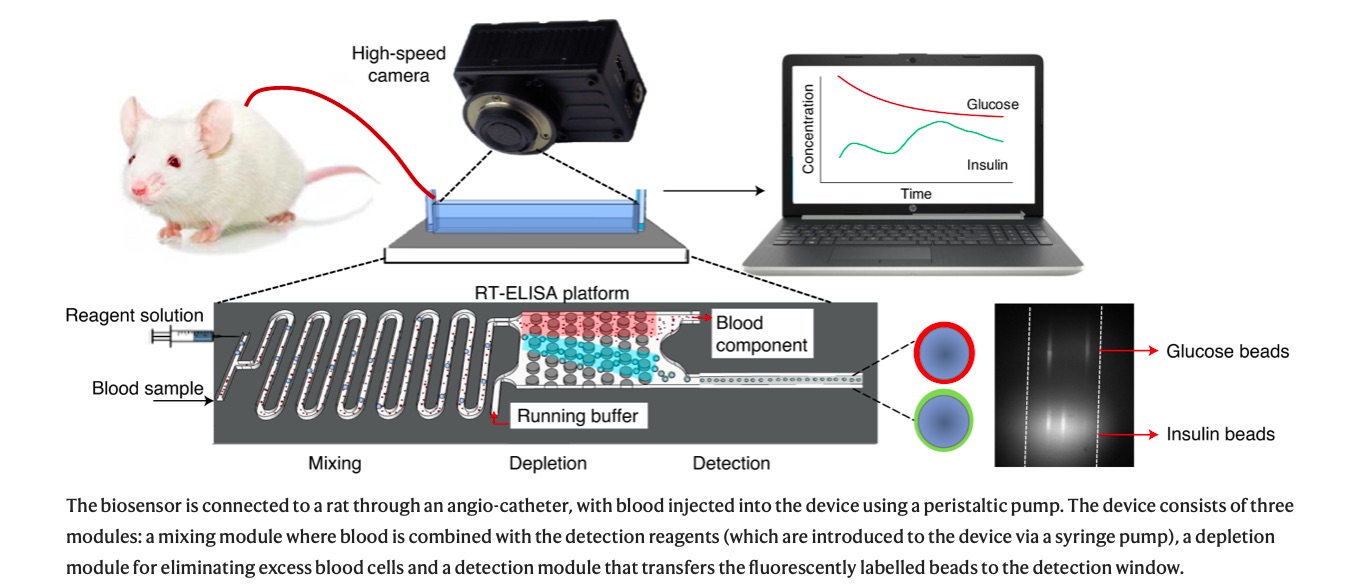
Knowing the rate at which a patient processes insulin is incredibly important, Maikawa explained. But because blood insulin is present in only very low quantities in diabetic patients, most continuous monitoring devices cannot measure it. Instead, patients use continuous glucose monitors, which measure the concentration of glucose in interstitial fluid, or the fluid in between blood vessels and cells, rather than the blood itself, to indirectly monitor the passage of insulin through their body.
“The interstitial fluid has about a delay of about 15 minutes from actual glucose concentrations in the blood,” Maikawa said. “So, the concentrations you’re seeing in these continuous glucose monitors is slightly delayed.”
By contrast, using the RT-ELISA, researchers do not have to account for any delay because they “are measuring the exact [glucose] concentrations in the blood that were present at that time,” Maikawa said.
Because there is no delay, Soh said that the RT-ELISA “is really well-suited for in-hospital use” because of doctors’ “direct access to the blood” and the continuous monitoring properties of the device.
Additionally, because of the RT-ELISA’s ability to detect substances at tiny concentrations, the device can directly monitor the rate at which a patient processes insulin.
Currently, patient insulin absorption rates are estimated using a population curve, but these estimates are not always accurate.
“Injection site, injection technique, or even things like temperature can change how quickly insulin is absorbed in a single individual at various times of the day,” Maikawa said. Differences between patients can also affect insulin absorption rate. The RT-ELISA’s monitoring abilities could help patients adjust their dosing schedule to fit their body’s unique absorption rate.
Looking toward the future
Rayhan Lal, an endocrinologist (not affiliated with the study) and member of the Stanford Diabetes Research Center — and Type 1 diabetic himself — explained that when he was diagnosed, the way to measure blood sugar levels was to “take a very unfortunately sized needle, stab your finger, put the blood on a strip, wait about a minute … and if you’re lucky, the meter would remember what the last blood sugar it had checked was.”
Calling continuous glucose monitors “a real quality of life improvement,” Lal said that one of the eventual benefits of the technology developed by Soh and his colleagues could be using it as a “fault detection tool” to determine whether population curves are accurate.
For patients with Type 2 diabetes, Lal explained that their bodies often make insulin, just not enough.
“In the Type 2 space, there’s also a need to know how much insulin is coming from the person’s body versus how much insulin is coming from what we asked them to inject,” he said.
“That’s one of the beauties of [Soh’s] technology,” he continued. “You might be able to differentiate those two because the amino acid sequence of what we inject is slightly different than what the body makes normally.”
While past research has primarily focused on diabetes markers like insulin and glucose, Soh reiterated that his current device is the “first generation” model of a biosensor. Soh said he hopes to use the second-generation model to track cytokine (a short-distance communication molecule) concentrations to better treat extremely sick patients. He hopes to develop further generations to provide earlier and more targeted treatment for other illnesses.
Soh said that the current healthcare landscape focuses on treatment after symptoms arise; pre-symptomatic treatment of many diseases, however, is often the best option. Devices similar to the RT-ELISA have the potential to increase pre-symptomatic treatment due to continuous monitoring that catches diseases before symptoms arise.
“As a society as a whole, it would behoove us to work on this side, rather than when things are completely out of control,” Soh said.
Even so, Lal cautioned against assuming people will actually use the continuous monitoring device. For example, someone with a history of a heritable cancer trait might be inclined to wear the device to catch the cancer early, but members of the general population might not be so willing.
One potential solution to this problem could be implementing the technology into devices that people already use. For example, Lal pointed out that Samsung and Apple are both working on developing a non-invasive glucose monitor that can be combined with smartwatches.
Soh said the main focus at this point remains on diabetes patients, those with neurodegenerative diseases and in-hospital use on extremely sick patients. But he is hopeful that one day research will advance beyond simply responding to illness and will begin “creating a technology that can constantly act as a sentinel.”
Contact Sofia Scekic at sscekic ‘at’ stanford.edu and Sarah Lewis at sarahelewis956 ‘at’ gmail.com.
Stanford researchers develop real-time biosensor for continuous blood concentration monitoring - by Sofia Scekic and Sarah Lewis - The Stanford Daily - February 8, 2021


